Menon Chandana, Mathew Josey, George Liza, Paul Sinju, Yacob Jeason Paul, Rajan Rishi
Department of Conservative Dentistry and Endodontics, Annoor Dental College and Hospital, Muvattupuzha
Running title – Antibacterial efficacy of Hydrogen water
Received: 09-05–2023
Revised: 15-05-2023
Accepted: 17-05-2023
Address for correspondence: Dr Chandana Menon, Parayil House, Sree Lakshmi
Elamakkara PO, Kochi-682026, Kerala
Email : cmenon2197@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Noncommercial ShareAlike 4.0 license, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms
How to cite this article: Menon C, Mathew J, George L, Paul S, Yacob J P, Rajan R. Comparative evaluation of Antibacterial efficacy of a novel root canal irrigant-Hydrogen water with conventional irrigants Sodium hypochlorite and Chlorhexidine gluconate : An In vitro Study. J Oral Biomed Sci 2023; 2(2):65-70
Abstract
Background: Endodontic infections have complex microbial flora including E-Faecalis .Root canal treatment attempts to eliminate microbial flora from the root canals. Improved irrigants are needed for this. Hence, researchers are trying to develop newer irrigants with better properties. This study attempts to examine the antibacterial efficiency of a novel irrigant, hydrogen water (HW), toward E. faecalis while comparing it with the conventional irrigants, notably sodium hypochlorite (NaOCl) and chlorhexidine (CHX).
Materials and Methods: Top surface of Petri dishes containing Blood Agar is flooded with 100μl inoculum suspension of E. faecalis and desiccated for 15 minutes. Three equidistant wells punched, 50μl of irrigants were put into the wells followed by incubation at 37ºC for 14 days. Growth inhibition zones were measured at the end of day 2 and day 14. Paired t-test and One-way ANOVA was applied.
Results: The highest values of zones of inhibition were obtained for sodium hypochlorite followed by chlorhexidine gluconate and least by hydrogen water both on day 2 and day 14 with statistically significant difference. On comparison of zones of inhibition between between day 2 and day 14 of treatment within the group also showed a statistically significant difference.
Conclusion: Though hydrogen water has lesser antimicrobial potential in comparison with NaOCl and CHX, considering its advantages like biocompatibility. So HW may be considered as an alternative biocompatible irrigant in endodontics. Irrigant activation procedures may be employed to augment its antibacterial efficacy.
Keywords: Hydrogen water, E faecalis, Antibacterial effect
INTRODUCTION
The purpose of endodontic treatment is to exterminate all the live and necrosed tissues, bacteria, and microbial metabolites from the system of root canals.1Irrigants used in combination with mechanical instrumentation helps in dissolving tissue, abolition of debris, and disinfectingng the root canal system. Sodium hypochlorite has organic tissue dissolving capacity and vast spectrum of antibacterial qualities and hence it is regarded as the gold standard irrigant. Often chelation solutions are indicated as supplemental irrigant for smear layer elimination or to limit its development on the surface of dentin.1
Endodontic infections are mainly caused by Enterococcus faecalis, one of the most resistant species which is an anaerobic gram-positive cocci that is typically a commensal in the oral cavity and has exhibited outstanding adaptation to such conditions with low nutrition along with low oxygen levels and complicated ecosystem. In situations of primary endodontic infection, E. faecalis is more probable to be related to cases that are asymptomatic rather than symptomatic ones.2
Potent antibacterial action with minimum friction during mechanical instrumentation along with accessibility and complete pulp dissolution are among the key attribute of a successful root canal irrigant. Sodium hypochlorite has largely been identified as an endodontic irrigant since its application was originally described in 1936 by Walker. It chiefly operates as an efficient antibacterial agent by excellent dissolution of living, necrotic, and fixed tissue. In spite of its appealing features, including ease of access and low cost, it has a few disadvantages, like a bitter taste, a tendency to bleach clothes and being possibly acidic. There is also a concern with regard to its destructive effects if extreme concentration of solutions are accidentally forced into the periapical tissues. Some arguments do exist pertaining to its antibacterial efficacy at lesser dosages, which are touted as an effort to decrease its detrimental impact.2
Chlorhexidine gluconate (CHX) has also been used for the disinfection of root canals. It has been proven that CHX exhibits powerful and substantial antibacterial action against the resistant Enterococcus faecalis. Although CHX is an admissible bacteriocidal agent, it is unable to dissolve the residual pulp tissues after the preparation of root canal. Since neither of the presently known irrigants fulfil all the parameters of an ideal irrigant, efforts are on to find an alternative biocompatible irrigant.3
Natural substances like hydrogen water have favorable antibacterial effects on oral microorganisms. It has exhibited antibacterial effectiveness against standard isolates of
- nucleatum, Actinobacillus actinomycetemcomitans,P.intermedia,P. gingivalis and the Treponema denticola species. Antibacterial efficiency of hydrogen water as root canal irrigant has never been examined in endodontics.4
MATERIALS AND METHODS
Production of hydrogen water
Hydrogen water bottle was commercially procured (GOMAX-H7, Yongkang Industry, China).
It consisted of electrodes at the base that aided in the synthesis of hydrogen water, and distilled water was put into the hydrogen water bottle for it to undergo the process of electrolysis. Electrolysis started in 3 seconds after the electrical supply of the bottle was turned on. This decomposition reaction resulted in liberation of hydrogen (H2) at the cathode. Consequently after 5 min, hydrogen water was freshly generated. 4
Methodology
In this investigation sodium hypochlorite, chlorhexidine and hydrogen water were taken for comparison of antibacterial effectiveness against E. faecalis. Strains were cultured from frozen stock cultures under aerobic conditions at 37°C for 24 hours of incubation in brain-heart infusion broth and assayed.Turbidity changes were noted which was indicative of microbial growth.Sterile saline was added to this for dilution so as to obtain a suspension of 0.5 turbidity on the McFarland scale.
The E. facecalis strain was treated to these irrigants for two lengths of time: 2 days and 2 weeks. The antibacterial effect of the irrigants was investigated by agar-well diffusion test.
Agar-well diffusion experiments were done on Petri dishes of 90-mm-diameter with nutrient agar at 4mm depth. 100μl inoculum solution of the strain was poured onto the top surface of agar plate and then dried for 15 minutes at a temperature of 37°C . Three equidistant wells with a diameter of 5mm and depth of 4mm were drilled in the agar plate using a Pasteur pipette following which 50μl of each irrigant were inserted into the plate’s well. The agar media was cultured for 2 weeks aerobically at 37°C. The complete experiment was replicated to reach a sample size of 42.
The sizes of growth inhibition zones were evaluated on Day 2 and Day 14 for comparison. Growth inhibition zones surrounding each irrigant were evident by the absence of bacterial colonies seen as clearing of agar in proximity to each agar well, and they were measured using a millimeter ruler. All of the values exceeding 6 mm were assessed as demonstrating a notable reduction in the growth of bacteria. The inhibitory zones with higher width were indicative of increased antibacterial activity of the irrigants.
Statistical procedure
The acquired data was analyzed using statistical software, SPSS (version 24.0.0.0). Comparisons between each group were done using one-way ANOVA and paired t-tests.
RESULTS
The zones of inhibition exhibited by each irrigants on the agar plate against E faecalis was recorded. On comparing the antibacterial efficacy of irrigants on Day 2 and Day 14, the mean values were higher on Day 14 than Day 2 for all the 3 irrigants (Table 1, Graph 1)
The mean values of the zones of inhibition were higher during 14 days for all the three root canal irrigants – Sodium Hypochlorite, Chlorhexidine and Hydrogen water when compared to day 2. This difference between day 14 and day 2 was statistically significant for all the irrigants. (p<0.001) (Table 2The highest mean value of the zone of inhibition was exhibited by sodium hypochlorite on both the days.
Comparison between the groups showed that there is significant difference in mean values of zones of inhibition with day 2 and day 14 among the groups. (p<0.001) (Table 3)
The study showed a higher mean values of zones of inhibition for sodium hypochlorite indicating the highest antibacterial efficacy of sodium hypochlorite on both Day 2 and Day 14 in comparison to other irrigants.
Chlorhexidine follows sodium hypochlorite in its antibacterial efficacy and hydrogen water exhibited the least antibacterial activity against E. faecalis.
DISCUSSION
Removal of damaged tissue, elimination of microorganism from the root canal system, and prevention of recontamination is considered as a crucial feature of endodontic therapy. It is necessary for the clinicians to be highly educated and have skill in this key component of endodontics. Chemomechanical preparation and profuse irrigation is vital to minimize the number of microbial flora inside the root canal to prevent periapical disease. It is also required for endodontic disinfection to be straightforward and cost-effective.
The result of this study shows the highest antimicrobial efficacy for sodium hypochlorite in comparison to other irrigants, This is in conformity with a research done by Arias et al., who demonstrated greatest antibacterial activity for 2.5% NaOCl against E. facecalis when used alone as well as in combination with HEBP.13 The antibacterial action is connected to better pulpal dissolution since sodium hypochlorite is highly basic in nature with pH of about 11 and it functions as a solvent for organic tissue, encouraging amino acid breakdown and hydrolysis via the formation of chloramine molecules.14 Though the antibacterial and tissue disintegration activity of hypochlorite grows with its concentration, there is a possibility of apical extrusion and toxicity.15
The antibacterial efficacy of sodium hypochlorite was followed by CHX in this research. 2% is recommended for mechanical irrigation of root canal. Previous studies reveal that it is strongly antibacterial, notably at pH 5.5–7.0. Chlorhexidine killed bacteria by a procedure in which the cell wall of bacteria was destroyed, resulting in a substantial drop in the colony viability of E. faecalis. Earlier research has proved that effective elimination of E. faecalis occurred from the outermost portions of the dentinal tubules when 2%CHX was used which is in accordance with our data. 8 Evans et al. established in their research that coupling of 1–2% CHX with calcium hydroxide resulted in effective eradication of E.faecalis. (R)
The key benefits of chlorhexidine are its decreased cytotoxicity and the absence of an unpleasant smell and unacceptable taste. Despite the features of chlorhexidine as an irrigating solution, it cannot be employed as a gold standard endodontic irrigant due to its failure to dissolve necrotic tissue. 15
It is expected that this research may uncover potential natural compounds with antibacterial effects in the field of endodontics. Since hydrogen water is one such natural molecule with antibacterial potential, it was chosen for this study. The findings of this investigation indicated lower antibacterial effectiveness for hydrogen water in contrast to sodium hypochlorite and chlorhexidine. This conclusion was contradictory to the study done by Aarati et al., who observed that hydrogen water demonstrated antibacterial action against clinical plaque specimens from individuals with chronic periodontitis. 16
The antibacterial capacity of the hydrogen water employed in this experiment is dependent on the products created during electrolysis. These products increased the bacterial cell membrane’s permeability thereby causing leakage of cellular components and annihilation of the bacteria. This is in keeping with work done by Zeng et al, who suggested the antibacterial mechanism of electrolyzed water processed against Staphylococcus aureus and Escherichia coli. (R)
The poor efficiency of hydrogen water against E. faecalis in our experiment can be attributed to its high pathogenicity and tough cell wall, and its capability to endure intra-canal treatments without the synergistic effects of other microorganisms. They have particular qualities that allow them to avoid instrumentation during endodontic treatment. These traits include the capacity to generate biofilms and emerge in complex locations distinct from the main canals, such as auxiliary canals, isthmuses and apical deltas. In addition, E. faecalis adopts several tactics for survival in severe settings. Those options include activating certain surviving genes, adopting different metabolic pathways, residing in a place with huge quantities of nutrients, and displaying synergism and bacterial aggregation skills.6
CONCLUSION
Endodontic lesions do not develop in the absence of bacteria. So it is imperative to debride the root canal system effectively to eliminate the microorganisms. In our study,though hydrogen water exhibited least antimicrobial potential in comparison to sodium hypochlorite and CHX, it should be considered for use due to its advantages like biocompatibility,and absence of bad taste and odour. Furthermore, it is considered to be safe if accidental apical extrusion occurs. The antibacterial potency of hydrogen water can be augmented through various irrigant activation systems. Further investigations regarding this are required to obtain a conclusive evidence.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Source of support: Nil
REFERENCES
1.Topbas C, Adiguzel O. Endodontic Irrigation Solutions: A Review. Int Dent Res 2017;7:54-61.
2.Luddin N, Aly Ahmed HM. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16:9–16.
3.Homayouni H, Majd NM, Zohrehei H, Mosavari B, Adel M, Dajmar R, et al. The Effect of Root Canal Irrigation with Combination of Sodium Hypo-chlorite and Chlorhexidine Gluconate on the Sealing Ability of Obturation Materials. Open Dent J. 2014;8:184–7.
4.Sundaram G, Ramakrishnan T, Parthasarathy H, Raja M, Raj S. Fenugreek, diabetes, and periodontal disease: A cross-link of sorts J Indian Soc Periodontol. 2018;22:122-126.
5.Ganesh Kumar A, Joseph B, Nandagopal S, Sankarganesh P, Jagdish SK. Experimental human root canal irrigant NaOCl against Enterococcus faecalis and 3T3, and determination of cytotoxicity effect. Biomed Pharmacol J. 2019;12:965–74.
6.Alghamdi F, Shakir M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus. 2020;12:e7257.
7.Iqbal A. Antimicrobial Irrigants in the Endodontic Therapy. Int J Health Sci (Qassim). 2012;6:1–7.
8.Dewiyani S, Bachtiar BM, Bachtiar EW, Sumawinata N. Antimicrobial Efficacy of Various Concentrations of Chlorhexidine Against Enterococcus Faecalis Bacteria. J Clin Diagnostic Res. 2019;1–4.
9.Solovyeva AM, Dummer PMH. Cleaning effectiveness of root canal irrigation with electrochemically activated anolyte and catholyte solutions: A pilot study. Int Endod J. 2000;33:494–504.
10.Tamaki N, Orihuela-Campos RC, Fukui M, Ito HO. Hydrogen-Rich Water Intake Accelerates Oral Palatal Wound Healing via Activation of the Nrf2/Antioxidant Defense Pathways in a Rat Model. Oxid Med Cell Longev. 2016;:26798423.
11.Kasuyama K, Tomofuji T, Ekuni D, Tamaki N, Azuma T, Irie K, et al. Hydrogen-rich water attenuates experimental periodontitis in a rat model. J Clin Periodontol. 2011;38:1085–90.
12.Mahesh M, Pillai R, Varghese NO, Salim AA, Murali N, Nair SS. An in vitro study of comparative evaluation of efficacy of electrochemically activated water as a root canal irrigant in smear layer removal. J Conserv Dent. 2020;23:447–50.
13.Arias-Moliz MT, Ordinola-Zapata R, Baca P, Ruiz-Linares M, García García E, Hungaro Duarte MA, et al. Antimicrobial activity of Chlorhexidine, Peracetic acid and Sodium hypochlorite/etidronate irrigant solutions against Enterococcus faecalis biofilms. Int Endod J. 2015;48:1188–93.
14.Zhang W, Torabinejad M, Li Y. Evaluation of cytotoxicity of MTAD using the MTT-tetrazolium method. J Endod. 2003;29:654–7.
15.Abraham S, Raj JD, Venugopal M. Endodontic irrigants: A comprehensive review. J Pharm Sci Res. 2015;7:5–9.
16.Nayak A, Bhatt A, Bhat K, Nayak R, Hooli A, Naik S. Assessment of antibacterial effect of hydrogen water on plaque from patients with chronic periodontitis. J Indian Soc Periodontol. 2021;25:193-196.
| NaOCl |
| CHX |
| Hydrogen water |
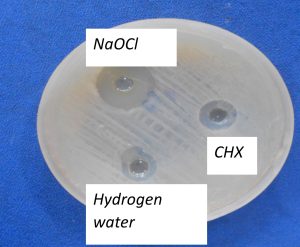
Fig 1 – Zone of inhibition of the irrigants on agar pate
| Group | Mean | N | Std. Deviation | Std. Error Mean | |
| Sodium Hypochlorite | Day 2 | 4.962 | 42 | 0.091 | 0.014 |
| Day 14 | 5.133 | 42 | 0.103 | 0.016 | |
| Chlorhexidine | Day 2 | 3.640 | 42 | 0.077 | 0.012 |
| Day 14 | 3.871 | 42 | 0.074 | 0.011 | |
| Hydrogen water | Day 2 | 1.464 | 42 | 0.174 | 0.027 |
| Day 14 | 3.740 | 42 | 0.066 | 0.010 |
Table 1: Mean values of irrigants on Day2 and Day 14
| Group | Day | Mean | Std. Deviation | Std. Error Mean | t | df | Sig. (2-tailed) |
| Sodium Hypochlorite | 2-14 | -0.171 | 0.152 | 0.023 | -7.315 | 41 | 0.000 |
| Chlorhexidine | 2-14 | -0.231 | 0.109 | 0.017 | -13.695 | 41 | 0.000 |
| Hydrogen water | 2-14 | -2.276 | 0.186 | 0.029 | -79.371 | 41 | 0.000 |
Table 2: Mean difference of irrigants on Day 2 and Day 14
| Sum of Squares | df | Mean Square | F | Sig. | ||
| Day 2 | Between Groups | 262.014 | 2 | 131.007 | 8870.030 | 0.000 |
| Within Groups | 1.817 | 123 | 0.015 | |||
| Total | 263.831 | 125 | ||||
| Day 14 | Between Groups | 49.694 | 2 | 24.847 | 3637.312 | 0.000 |
| Within Groups | 0.840 | 123 | 0.007 | |||
| Total | 50.535 | 125 |
Table 3 : Intragroup mean values of irrigants

