Thomas Priya1, Ramani Pratibha2, Abilasha R2
1 – Department of Oral and Maxillofacial Pathology Annoor Dental College & Hospital, Muvattupuzha, Kerala
2 – Department of Oral and Maxillofacial Pathology, Saveetha Dental College and Hospital, Saveetha University, Chennai
Received: 11-02-2023
Revised: 17-02-2023
Accepted: 27-02-2023
Address for correspondence: Dr. Priya Thomas, Department of Oral and Maxillofacial Pathology, Annoor Dental College & Hospital, Muvattupuzha, Ernakulam dist, Kerala-686673, India
Email – priyathomask@gmail.com
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Noncommercial ShareAlike 4.0 license, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms
How to cite this article: Thomas P, Ramani P, Abilasha R. Comparison of Calretinin Expression in Ameloblastoma, Odontogenic Keratocyst and Oral Epithelial Dysplasia: An Immunohistochemical Study. J Oral Biomed Sci 2022; 1:18-24
Abstract
Introduction: Oral epithelial dysplasia is a potentially precancerous lesion diagnosed histologically. While the risk of progression is associated with histological grade, it is currently impossible to predict accurately which lesions will progress. More accurate markers predicting progression to cancer would enable the targeting of these lesions for more aggressive treatment and closer follow-up. Calretinin is a calcium-binding protein involved in calcium signaling with a wide spread distribution in normal and neoplastic tissues. It is a well-established marker for epithelial mesothelioma and ameloblastoma and expression has been described increasingly in other neoplasms. Objective: The objective of this study was to determine the expression of calretinin in oral epithelial dysplasia and its possible specificity as an immunohistochemical marker in grading of dysplasia. Methodology: Fifteen cases of oral epithelial dysplasia, of which five were mild dysplasia, five moderate and other five of severe dysplasia, two cases each of acanthomatous ameloblastoma, KCOT, lipoma and normal epithelium were studied. Sections were immunohistochemically stained with calretinin antiserum using a standard avidin±biotinylated peroxidase complex method. The slides were analyzed for positivity to the marker and its varying expression through the three grades of dysplasia. Results: Negative staining was seen in 15 cases of oral epithelial dysplasia while the staining was positive and of moderate intensity in acanthomatous ameloblastoma restricted to the stellate reticulum-like epithelium especially in areas of squamous metaplasia. The other cases gave a negative effect on immnunohistochemical staining by calretinin. Conclusion: The biological significance of calretinin expression in tumourogenesis and premalignancy is not known and its use as a distinctive, specific immunohistochemical marker for dysplastic cells remains to be confirmed
Keywords: calretinin; oral epithelial dysplasia, ameloblastoma
Introduction
Calretinin is a 29-kDa vitamin D-dependent calcium-binding protein specific to the nervous system, named so on its initial characterization in retina, and involved in calcium signalling. The calretinin gene was first isolated from a cDNA clone from the chick retina and shows 60% homology with the calbindin1.
Being a cell specific protein, it is abundantly expressed in central and peripheral neural tissues, present within the subsets of neurons throughout the brain and spinal cord, in the neurons of sensory pathways, retina 1, 2, germinal epithelium of the ovary3 the adrenal glands,4 tendon fibroblasts,5 Leydig cells,6 thymus7 and many pathological human tissues. It is a member of the large family of EF-hand proteins. The EF-hand homologue family contains more than 200 different calcium-binding proteins; among them are calretinin, calmodulins, troponin C, myosin-regulatory light chain, parvalbumin, S-100 proteins and calbindins 9 and 28 kDa. EF-hand proteins are characterized by a peculiar amino acid sequence that folds up into a helix-loop-helix which acts as the calcium-binding site; calretinin contains six such EF-hand stretches.8
The exact biological function of calretinin remains unknown but its role as a calcium buffer preventing an abnormal intracellular calcium increase 9,10,11, calcium modulators and regulator of apoptosis have been postulated.12 Recent studies on transgenic mice have also revealed that these molecules play pivotal roles in Ca2+ homeostasis and signalling. The expression of calretinin, calmodulin, parvalbumin and calbindin has been of particular interest in the field of neuro-anatomy.10 However, its expression outside the nervous system has been less extensively characterized and many studies have been performed on non-human tissues like rat brain. In the tooth pulp, myelinated and unmyelinated fibers of the tooth pulp were found to be calretinin immunoreactive fibers, which projected as varicose terminals to the subodontoblastic and odontoblastic layers. 13
Calretinin has been identified as a definitive marker for mesotheliomas in the immunohistochemistry panel, and has also been demonstrated in some carcinomas and adenocarcinomas of lung, breast, pancreas, ovary and other tumours like ameloblastoma. In some tumors, calretinin immunoreactivity appeared to be acquired following neoplastic transformation. 14 These binding proteins have been expressed in squamous metaplasia of acantomatous ameloblastoma. Calretinin is also expressed in mast cells and mast cell lesions of the skin.15
Epithelial dysplasia may be morphological phenotypes of different steps in the progression from normal to malignant tissue. Malignancy, a two-step process with the presence of an initial precursor lesion and its subsequent potentiality to develop into a carcinoma has been recognized. The presence of epithelial dysplasia is commonly accepted as one of the most important predictors of malignant transformation in potentially malignant disorders. Dysplasia carries a high risk of malignant change. More severe the grade of dysplasia, greater is the risk of malignant change.
Many diagnostic and prognostic markers have been proved its significance in dysplasia. However, these calcium-binding proteins have not been demonstrated in oral epithelial dysplasia. The aim of this study was to investigate calretinin expression in oral epithelial dysplasia and its possible specificity as an immunohistochemical marker in grading of dysplasia.
Materials and methods
Fifteen cases of oral epithelial dysplasia, of which five were mild dysplasia, five moderate and other five of severe dysplasia, two cases each of acanthomatous ameloblastoma, KCOT, lipoma and normal epithelium were retrieved from the archives of Department of Oral Pathology, Saveetha Dental College and Hospitals. The cases were confirmed on the basis of adequate clinical, radiological, and histopathological diagnosis.
Immunohistochemistry
Immunohistochemical analysis was performed on 3µ paraffin embedded tissue sections on gelatin coated glass slides. After heat drying, sections were de-paraffinized in xylene and subsequently rehydrated in gradients of ethanol. Antigen retrieval to unmask antigenic sites was done using 10mM citric acid solution (pH 6) followed by a washing step with Tris buffered saline (TBS pH 7.6). Further incubations with pre-diluted ready to use primary mouse polyclonal antibody anti Calretinin (Biogenex, San Ramon, CA, USA) was performed at 37o C for 30 minutes. Sections were washed again and incubated with biotinylated secondary antibody (DAKO, Carpenteria, CA, USA) for 30 minutes followed by the streptavidin-biotin-peroxidase (DAKO, Carpenteria, CA, USA) for 30 minutes at room temperature. Colored reactions were developed by incubating with 3’3’–diaminobenzidine (0.05 diaminobenzidine in 0.05M Tris buffer, pH 7.6, and 0.01 H2O2) and subsequently counterstained with Harris hematoxylin and mounted with DPX (Dysterene Plasticizer Xylene). Positive and negative controls were included in all reactions. The positive controls used were two cases of rat brain and goat cerebellum (Figure 1a-1d, Table 1). Negative controls were performed by substituting the primary antibody with non-immune serum. Presence of brown colored reaction localized to the nucleus or cytoplasm was considered as positive reaction. The intensity of the immuno-staining for Calretinin was classified as negative, weak or strong in a blinded analysis performed by two independent pathologists.
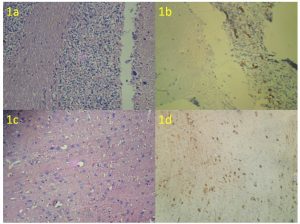
Figure 1a & 1b: H & E of goat cerebellum, Positive control (IHC- Calretinin)
Figure 1c & 1d: H & E of rat brain, Positive control (IHC- Calretinin)
Table 1: Expression of Calretinin in Controls

Results
All 15 cases of oral epithelial dysplasia showed negative expression of calretinin, both in the epithelial and stromal components (Figure 2a, b & c). Areas of squamous metaplasia within the stellate reticulum-like epithelium of acanthomatous ameloblastoma showed prominent immunoreactivity (Figure 2d).
All the cases of KCOT, lipoma was negative in all cyst wall components and stromal components respectively (Table 2).
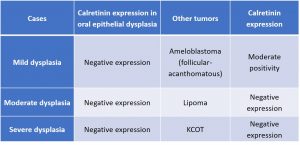
Table 2: Expression of Calretinin in Oral Epithelial Dysplasia in comparison with other cases
Figure 2a, 2b, 2c – Negative expression of calretinin in mild, moderate and severe dysplasia
Figure 2d: Mild positivity in focal areas of stellate reticulum of acanthomatous ameloblastoma
Discussion:
Calretinin has emerged as a high discrimination immunohistochemical marker in specific areas of pathology. Its expression has also been investigated in many normal human tissues and other human neoplasms but its potential role as a specific immunohistochemical marker of these tissues has yet to be elucidated. Only a partial correlation between staining of normal cells and their neoplastic counterparts has been observed.
Epithelial dysplasia is a premalignant lesion of stratified squamous epithelium characterized by histologic changes that include cellular atypia and loss of normal maturation and stratification. Incipient or early dysplastic changes are usually limited to the basal and parabasal layers of the epithelium. Epithelial dysplasia indicates an increased risk with the subsequent development of squamous cell carcinoma. To the best of our knowledge there are no previously published reports on calretinin expression in oral epithelial dysplasia.
Numerous studies have been carried out with odontogenic tumours and cysts. Rudraraju A et al. investigated the expression of calretinin in various types of odontogenic cysts and tumors, including ameloblastomas, odontogenic keratocysts, and dentigerous cysts. They found that all ameloblastomas (n=15) showed strong and diffuse expression of calretinin, whereas none of the other tumors or cysts showed positivity for calretinin. The authors also discussed the possible role of calretinin in the pathogenesis of ameloblastoma, suggesting that calretinin may play a role in the differentiation of ameloblastoma cells into functional ameloblasts. They concluded that calretinin could be a potential diagnostic marker for ameloblastoma and may provide insights into the pathogenesis of this tumor 16.
Sunjaya HA et al. 2021, compared the expression of calretinin in dentigerous cysts, odontogenic keratocysts, and unicystic ameloblastomas. They analyzed the expression of calretinin and found that all the samples of unicystic ameloblastoma (n=10) showed strong and diffuse calretinin expression, while only samples of odontogenic keratocyst (n=5) showed negative expression and dentigerous cyst (n=5) showed weak and focal calretinin expression. This was similar to demonstration of OKC cases in our study. 17
The authors concluded that calretinin could be a potential diagnostic marker for unicystic ameloblastoma and that its expression could help differentiate this tumor from other cystic lesions. However, they also noted the need for further studies with a larger sample size to confirm their findings and understand the role of calretinin in odontogenic tumors and cysts. In view of the wide spread distribution of calretinin in many normal and neoplastic human tissues and because of the demonstration of calcium-binding proteins in odontogenic epithelium during odontogenesis and in odontogenic tumours especially in squamous metaplasia of acathomatous ameloblastoma, it occurred to us that calretinin might also be expressed in dysplastic epithelium and could have some application in the differential diagnosis of various grades of dysplasia. Calretinin expression was particularly intense in the areas with squamous metaplasia of the stellate reticulum-like cells 18 (Table 3).
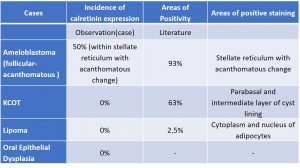
Table 3: Incidence and areas of expression of Calretinin in Study Cases in comparison with literature
This propensity for expression by squamous cells concurs with the documentation by Doglioni et al.14 of calretinin expression in keratinizing epithelial cells of the thymus and in the superficial layer of the pilar infundibulum. However, only one of the squamous carcinomas in their series stained positively. As evidenced by literature about 22.2% of SCC of oral cavity has shown weak positivity for calretinin expression and none have shown strong positivity. 19
Calcium has been known to play an important role in tumorogenesis. Calretinin being a calcium binding protein, and epithelial dysplasia being the prior state of progression to squamous cell carcinoma, we thought calretinin might have some role in dysplasia
With this in mind and in correlation with the previous studies, the thought of expression of calretinin in squamous cells and thereby its relation and expression in epithelial dysplasia and the role of calcium binding proteins in the initial stages of tumouriogenesis was to be elucidated.
The possible mechanism of positive calretinin expression outlined in case of ameloblastomas is due to an increase in calcium ions which acts as second messengers that lead to an increased proliferation and differentiation and metaplasia of stellate reticulum like cells. Calretinin being a calcium modulator tend to absorb increased intracellular calcium. It has been previously demonstrated that calretinin may act as an anti-apoptotic factor.20 Stellate reticulum, a pro-apoptotic differentiating region would suggest for the increased calretinin expression. 21
The intracellular calcium ions (Ca2+) are considered to be important second messengers that regulate various cellular processes like muscle contraction, synaptic transmission, cellular proliferation, apoptosis etc by activating or inhibiting cellular signalling pathways and Ca2+- regulated proteins. Although its exact function is still unknown, it has been recently shown that it participates in the progress of the cell cycle of certain malignant tumour cells 19,.22 and that its expression varies according to the state of differentiation of these cells.23 Calcium influences epithelial differentiation by altering the transcription of target genes and by regulating activity of enzymes critical in epithelial differentiation, such as transglutaminases proteinases, and protein kinases. In vitro studies also have shown that alteration of intracellular calcium pools can influence differentiation (Li et al., 1995) Calcium plays a role in vivo is the presence of a calcium gradient in epidermis that increases from the basal to the granular cell layer.
Some Ca2+- mediated signalling pathways are implicated in tumorigenesis and tumour progression, namely metastasis, invasion and angiogenesis.24 Calcium binding proteins act as mediators for their signal transmission. Ca2+ pumps and channels with altered expression in cancer might represent potential biomarkers of disease. 24
Though absent in normal epithelium, calretinin immunoreactivity appeared to be acquired following neoplastic transformation in many tumours, as documented by Doglioni et al (1996)14. Since dysplasia is characterized by the loss of maturation, this could hold a possibility for the negative expression. Dynamic spatial and temporal distribution exhibited by calretinin is that this protein too, as suggested for calbindin by Elms and Taylor (1987)25, may be present only in cells that reside directly in the path of calcium in transition, acting as a ‘calcium ferry’. Calretinin expression varies according to the metabolic activity of the cell and may be lost when this activity is altered or changes.
Conclusion:
The amplitude, temporal and spatial expression of calcium is regulated to achieve special outcome like cell cycle, apoptosis or proliferation of the cell. Since calcium plays a major role in the progression of tumour further insight is needed to evaluate the expression and effect of calretinin as a calcium binding protein in dysplasia and in tumorigenesis.
Conflict of interest: Nil
Financial support: Nil
References:
1. Andressen C, Blumcke I, Celio MR. Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res. 1993; 271:181-208.
2. Schwaller B, Buchwald P, Blumcke I, Celio MR, Hunziker W: Characterisation of a polyclonal antiserum against purified human recombinant calcium binding protein calretinin. Cell Calcium: 1993; 14: 639-648.
3. Bertschy S, Genton CY, Gotzos V: Selective immunocytochemical localization of calretinin in the human ovary. Histochem Cell Biol 1998; 109: 59-66.
4. Afework M, Burnstock G: Calretinin immunoreactivity in adrenal glands of developing, adult and aging Sprague-Dawley rats. Int J Dev Neurosci 1995; 13:515-521.
5. Gangji V, Krebs C, Rooze M, et al: Calretinin is transiently expressed in tendon fibroblasts of the paravertebral region of the chick embryo. Eur J Morphol 1997; 35:137-142.
6. Strauss KI, Isaacs KR, Ha QN, et al: Calretinin is expressed in the Leydig cells of rat testis. Biochim Biophys Acta 994; 1219: 435-440.
7. Kiraly E, Celio MR: Parvalbumin and calretinin in the avian thymus. Anat Embryol (Berl) 1993; 188: 339-344.
8. Rogers J, Khan M, Ellis J. Calretinin and other CaBPs in the nervous system. Adv. Exp. Med. Biol.1990; 269: 195-203.
9 Dei Tos AP, Doglioni C. Calretinin: a novel tool for diagnostic immunohistochemistry. Adv. Anat. Pathol. 1998; 5: 61-66.
10. Rogers JH: Calretinin: A gene for a novel calcium-binding protein expressed principally in neurons. J Cell Biol 1987; 105: 1343-1353.
11. Parmentier M. Structure of the human cDNAs and gene coding for calbindin D28K and calretinin. In: Pochet, R., Lawson, D.E.M., Heizmann, C.W. (Eds.), Calcium Binding Proteins in Normal and Transformed Cells. Advances in Experimental Medicine and Biology. Plenum, New York, 1989; 27–34.
12. Parmentier M, Lefort A: Structure of the human brain calcium- binding protein calretinin and its expression in bacteria. Eur J Biochem 196:79-85, 1991
13.Dharmesh Mistry, Mario Altini, Hedley G. Coleman, Hasiena Ali, Eugenio Maiorano: The spatial and temporal expression of calretinin in developing rat molars (Rattus nor egicus): Archives of Oral Biology 2001; 46: 973–981.
14. Doglioni, C., Dei Tos, A.P., Laurino, L., Iuzzolino, P., Chiarelli, C., Celio, M.R., Viale, G. Calretinin: a novel immunohistochemical marker. 1996; 20:1037-46
15. Mangini J, Silverman JF, Dabbs DJ et al. Diagnostic value of calretinin in mast cell lesions of the skin. Int J Surg Pathol 2000; 8:119–22.
16. Rudraraju A, Venigalla A, Babburi S, Soujanya P, Subramanyam RV, Lakshmi KR. Calretinin expression in odontogenic cysts and odontogenic tumors and the possible role of calretinin in pathogenesis of ameloblastoma. Journal of Oral and Maxillofacial Pathology: JOMFP. 2019;23(3):349.
17. Sunjaya HA, Fatah WR, Rahadiani N, Vitria EE. Comparative Study of Calretinin Expression in Dentigerous Cyst, Odontogenic Keratocyst, and Unicystic Ameloblastoma. Journal of International Dental and Medical Research. 2021; 14(3):965-9.
18. Altini M, Coleman H, Doglioni C, Favia G & Maiorano E. Histopathology. 2000; 37: 27-32.
19. Alessandro Lugli, Yvonne Forster, Philippe Haas, Antoinio Nocito, Christoph Bucher, Heidi Bissig, Martina Mirlacher, Martina Storz, Michael J. Mihatsch,and Guido Sauter: Calretinin Expression In Human Normal And Neoplastic Tissues: A Tissue Microarray Analysis On 5233 Tissue Samples: Human Pathology 2003; 34: No. 10
20. Gander Jc, Gotzos V, Fellay B, Schwaller B. Inhibition of the proliferative cycle and apoptotic events in WiDr cells after downregulation of the calcium-binding protein calretinin using antisense oligodeoxynucleotides. Exp. Cell Res. 1996; 225: 399–410.
21. M.Alaeddini. Comparitive expression of calretinin in selected odontogenic tumours: a possible relationship to histiogenesis: Histopathology 2008; 52: 299-304.
22. Gotzos V, Schwaller B, Hetzel N, Bustos-Castillo M, Celio MR. Expression of the calcium binding protein calretinin in WiDr cells and its correlation to their cell cycle. Exp Cell Res. 1992; 202:292–302.
23. Cargnello R, Celio MR, Schwaller B, Gotzos V. Change of calretinin expression in the human colon adenocarcinoma cell line HT29 after differentiation. Biochim Biophys Acta 1996; 1313:201–208
24. Gregory R. Monteith, Damara McAndrew, Helen M. Faddy and Sarah J. Roberts-Thomson: Calcium and cancer: targeting Ca2+ transport: Nature Reviews: Cancer. 2007; 7: 519
25. Elms TN, Taylor AN. Calbindin-D28K localization in rat molars during odontogenesis: J.Dent. Res. 1987; 66:1431-1434

